At the time, most paleontologists viewed the dinosaur extinction as a gradual event capped by the final extinctions at the end of the Cretaceous. To Alvarez, however, the KT boundary certainly looked catastrophic and sudden — but the timing of the event was still an open question: was the KT transition (represented by the clay layer in the stratigraphy) gradual or sudden? To answer that question, he needed to know how long it had taken to deposit the clay layer — but how could he time an event that happened 65 million years ago? Walter Alvarez discussed the question with his father, the physicist Luis Alvarez, who suggested using beryllium-10, which is laid down at a constant rate in sedimentary rocks and then radioactively decays. Perhaps beryllium could serve as a timer.
Their idea was to recruit Richard Muller, another physicist, to help measure the amount of beryllium-10 in the clay layer, correct for how much the beryllium would have decayed since then, and then reason backwards to figure out how many years would have had to pass for that much beryllium to be deposited. However, before they could take the beryllium measurements, they learned that the published decay rate for the isotope was wrong. Calculations based on the new numbers revealed that the planned analysis would not work. For the amounts of beryllium that they could detect, the timer in the 65 million year old clay layer would have already run out — all of the beryllium would have decayed away.
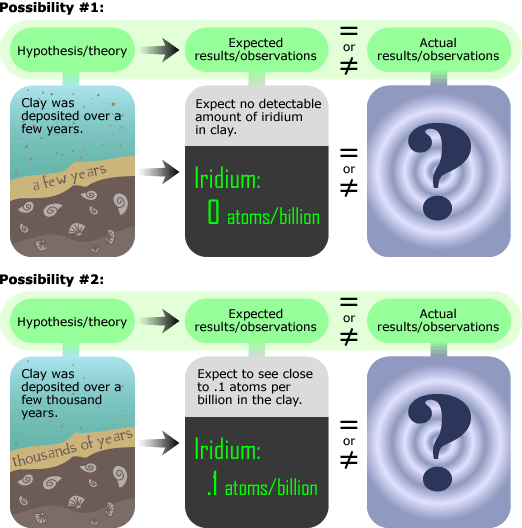
The beryllium investigation turned out to be a dead end, but Luis Alvarez soon came up with a replacement: iridium. Iridium is incredibly rare in the Earth’s crust but is more prevalent in meteorites and meteorite dust. They reasoned that since meteorite dust and hence, iridium, rain down upon Earth at a fairly constant rate, the amount of iridium in the clay would indicate how long it took for the layer to be deposited. An observation of more concentrated iridium (around one iridium atom per ten billion other particles) would have implied slower deposition, and less iridium (an undetectably small amount) would have implied rapid deposition and a sudden KT transition, as shown below.
Get ideas for teaching about radioactive decay from the Science Education Resource Center.