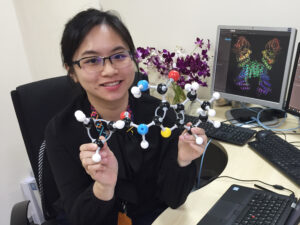
Crick and Watson wanted to work on DNA’s structure, but they couldn’t approach it as Wilkins and Franklin were — through X-ray diffraction. First, Crick was a friend of Wilkins and didn’t want to step on his toes. Second, Watson and Crick didn’t have the high-quality DNA samples necessary for X-ray diffraction. But Watson and Crick had another way of working — they could form hypotheses about DNA’s structure by building a physical model of how its atoms fit together.

Today, ball-and-stick models like the ones Watson and Crick used are available in most chemistry classes — but in 1951, they were found in only the best-equipped labs. In the first half of the 20th century, painstaking chemical work established the approximate sizes of atoms, the number of bonds they form with other atoms, and the angles at which these bonds form. The models Watson and Crick worked with incorporated all of this information. The flexibility and accuracy of the models allowed them to try out many different structures and quickly see whether they agreed with what was known about chemical bonding. This made the models a good way to form new hypotheses about the shape of a molecule — something too small to observe directly.
Watson and Crick were also encouraged by the fact that Linus Pauling, a chemist who studied bond formation, had just used models to figure out the helices that are part of the structures of many proteins. Pauling came up with the solution by starting with X-ray diffraction data, then using ball-and-stick model-building as a shortcut. This approach had allowed him to find the solution much more quickly than he could have by using X-ray data alone. The success of his approach inspired Watson and Crick to try the same thing.