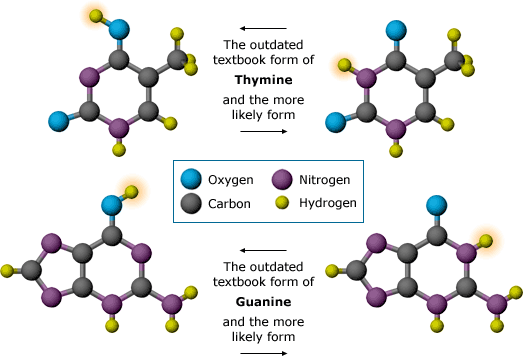
Watson and Crick were also stuck on what to do with the bases. At first, Watson thought they paired together A-A, C-C, T-T, G-G — but because of the different sizes of the bases, the hypothesis had to be discarded. It would have required a sugar-phosphate backbone that wiggled in and out, rather than winding around in smooth twists. Then, Watson and Crick got a key piece of evidence about the shapes of the bases from a visiting American chemist, Jerry Donohue. At that time, most chemistry textbooks reported a particular placement of hydrogen on the bases. That placement made it impossible to match A to T, or G to C — they just didn’t fit. Donohue told Watson that the textbooks were outdated. More was now known about the shapes these bases might take: one of the hydrogen atoms could be attached to the base in another location. In fact, based on a few different lines of evidence, Donohue thought that the bases likely took shapes that Watson had not yet tried.
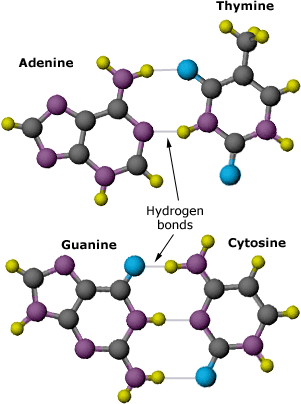
Watson tried to fit the new shapes into the two-chain model he and Crick had developed. On February 28th, he was playing with paper cutouts of each base when he suddenly saw the answer. The A fit with T, and G fit with C. Plus, the A-T pair had the exact same molecular length as the G-C pair! Bonded together like this, the bases wouldn’t bump and repel one another. Crick realized that if the bases paired up like this, it would explain the mysterious base ratios: A=T, G=C. Suddenly, it made perfect sense that the base pairs must be in the center of the molecule, and that the two sugar-phosphate strands wound around them. It even suggested how one strand could be used to copy the other. Because each base always matches up with the same partner, the order of bases on one strand could determine the exact order of bases on a new strand. Within a week, Watson and Crick had worked out the details of their hypothesis about the molecular structure of DNA.