The discovery of CFCs’ environmental impact began in 1970, in the unlikely setting of a vacation home on the bucolic west coast of Ireland. James Lovelock, a medical researcher turned self-employed scientist, wanted to know whether the haze obscuring the view from his home was natural or from human sources. He hypothesized that if pollution were causing the haze, then its source would be an urban area and it would contain large concentrations of synthetic chemicals. Since CFCs don’t occur naturally, Lovelock thought that looking for these chemicals in the air would be a good test. Using an instrument he’d designed himself, he detected CFCs in the haze, confirming its human-made origins.
However, what really piqued his curiosity were the results on clear days. According to his hypothesis, on clear days, when the air was coming from over the Atlantic without having passed over an urban area for thousands of miles, CFCs should be close to undetectable. Surprisingly, he was easily able to detect CFCs even on pristine days. Wanting to know if CFCs were building up in the atmosphere everywhere, Lovelock brought his instrument on a sea voyage from England to Antarctica, taking measurements all along the journey. Wherever he traveled, he found CFCs.
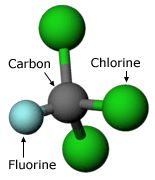
Lovelock presented his findings in 1972 at a scientific meeting that aimed to bring together meteorologists and chemists — two sets of researchers which, up to this point, had mixed very little. There, his observations caught the attention of Sherwood Rowland, a chemist at the University of California, Irvine. Rowland was curious about what happened to these chemicals once they were released into the atmosphere. Even very stable chemicals can react under the right conditions; for example, even stainless steel will react when it’s exposed to salty water and high temperatures. Rowland wanted to know what the right conditions were for CFCs to react and what effects this might have.