Rowland was joined in his investigation by the newest member of his research group — Mario Molina, a chemist from Mexico fresh out of graduate school. Rowland had suggested a few different topics for a first project and Molina thought that investigating the fate of CFCs released into the environment was the most interesting of the bunch. Excited to learn about a new field — atmospheric chemistry — Molina plunged right in. As is expected of a scientist, he began with a thorough review of the scientific literature on the subject; perhaps someone else had investigated a chemical reaction that would affect CFCs. He found that many chemicals are broken down in the lower atmosphere near where they are released — but not CFCs. No known chemical processes seemed to be able to affect CFCs in the lower atmosphere.
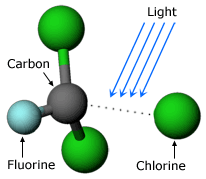
He wondered what might happen to CFCs as they drifted upwards. At low altitudes, much solar radiation has been filtered out by the atmosphere, but at high altitudes, solar radiation is much more intense. From his understanding of chemistry, Molina knew that once any molecule got high enough, strong solar radiation would break it apart. Using atmospheric scientists’ discoveries about air movement, Molina calculated that it would take somewhere between 40 and 150 years for a CFC molecule to randomly diffuse up to the height where it would be broken down by solar radiation, releasing a chlorine atom in the process.
To find out what would become of this chlorine atom, Molina searched through other scientists’ publications to see what atmospheric molecules would be near this chlorine atom when it split off. Among the many possibilities, one molecule stood out: ozone — three oxygen atoms linked together. Molina learned that chlorine would react catalytically with ozone — meaning that the chlorine atom could act like an axe, encouraging a reaction that chops up ozone without hurting the chlorine at all. In fact, a single chlorine atom could destroy around 100,000 ozone molecules! Molina wasn’t sure how big a difference this would make in the atmosphere, so he compared effects of CFCs to natural ozone depletion mechanisms investigated by other researchers. He found that CFCs could lead to even more ozone destruction than the natural mechanisms did!
Sounding the alarm
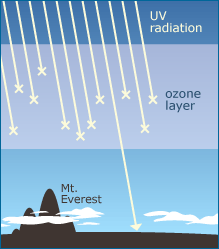
The ozone layer protects Earth from dangerous UV radiation — which can cause mutations. In humans, a depleted ozone layer would likely mean higher rates of skin cancer, cataracts, and immune system problems. Further, an increase in UV radiation could affect plants and marine ecosystems in unpredictable ways — which could, in turn, trigger other ecological changes. Because it seemed that CFCs could destroy our protective ozone shield, Molina and Rowland were alarmed! But they were also skeptical: if this ozone destruction were actually going on, why hadn’t atmospheric scientists discovered it already? After checking their calculations, they decided to consult a colleague in atmospheric chemistry and learned that, only a few months earlier, researchers had found the same chlorine-ozone interaction in the exhaust from space shuttles — a very small cause of ozone destruction compared to CFCs. After being assured that their findings warranted serious concern, Molina and Rowland published their work.1 Then, to increase the likelihood that action would be taken on these disturbing results, they took their findings to the news, media, and policymakers, calling for a ban on the production and use of CFCs. But they didn’t stop there …
Before diving into a new research area, Molina learned what other scientists had already discovered about the topic. This is a standard part of scientific behavior. To learn about other kinds of behavior expected of scientists, see Participants in science behave scientifically.
1Molina, M.J., and F.S. Rowland. 1974. Stratospheric sink for chlorofluoromethanes: chlorine atom-catalyzed destruction of ozone. Nature 249:810-812.