Things were looking good for Molina and Rowland’s hypothesis — but bad for the ozone layer. Their ideas now had supporting atmospheric evidence and were gaining acceptance within the scientific community. However, a hallmark of scientific thinking is skepticism — even towards your own hypotheses. Though it was supported by many lines of evidence, Molina and Rowland didn’t assume that their hypothesis was correct, but continued looking for processes in the atmosphere that might offset the effects of CFCs.
One possibility was nitrogen dioxide. Scientists knew that nitrogen dioxide could react with chlorine — the atom from CFCs that actually breaks down ozone — and potentially tie up the destructive atom in a harmless form: chlorine nitrate. However, Molina and Rowland hadn’t taken this reaction into account earlier because 1950s measurements had indicated that chlorine nitrate is short-lived — that is, shortly after it is formed, it will be broken down by sunlight, releasing the harmful chlorine back into the atmosphere. Now, Molina and Rowland decided to check those old measurements with more lab experiments. They found that chlorine nitrate stuck around much longer than previously thought and might be able to take chlorine atoms out of commission. This reaction needed to be considered in the atmospheric models. Even though these findings cast doubt on their hypothesis, they quickly brought this information to the attention of the scientific community, reporting and publishing their results.
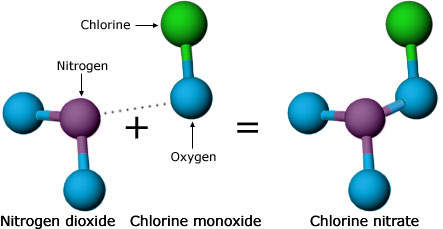
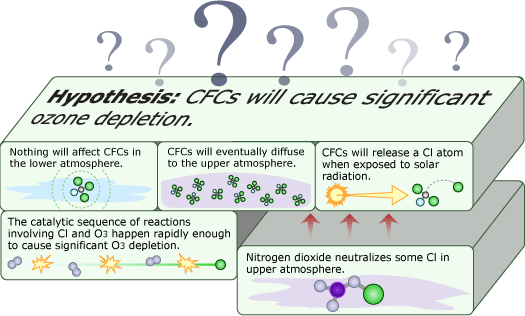
As researchers rushed to incorporate chlorine nitrate into their models, they encountered some surprises. Several groups were involved in testing the Rowland-Molina hypothesis, and up to this point, all of them had agreed on what should be observed in the atmosphere if Molina and Rowland were right. But once the modelers added the chlorine nitrate reactions to their models, different groups got conflicting results. Some models even predicted a net increase in ozone!
What could be going on? The problem was traced to an approximation used as a means of simplifying some of the models. Scientists try to limit the factors represented in models to the ones essential for their purposes. Often this is because overly complex models can require calculations that would take a computer, even a supercomputer, years to complete. Scientists use their background knowledge to try to figure out what simplifications might be appropriate for a particular model and then try to check their validity. These simplifications form another set of sub-hypotheses. If a model is inaccurate, it might be because one of the central ideas in the model is wrong, or it might be because one of its approximations gives an oversimplified result.
In the case of the atmospheric models, scientists found that those models predicting a net increase in ozone had one thing in common: they all used the approximation that the sun shines at its average intensity all the time, instead of varying throughout the day. This turned out to be an oversimplification — an incorrect sub-hypothesis. Removing this approximation brought these models into agreement with the other models and the actual atmospheric measurements of chlorine nitrate. Even with the chlorine nitrate reactions incorporated into the models, the Rowland-Molina hypothesis predicted significant (though lower) levels of ozone depletion.