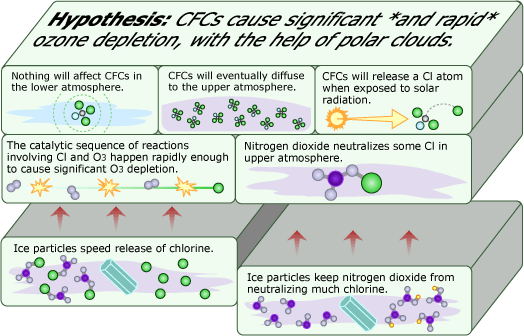
The shocking Antarctic ozone losses had many scientists intrigued, including atmospheric chemist Susan Solomon. Based on her expertise in modeling atmospheric chemistry and air movements, Solomon suspected that some unknown chemical processes involving CFCs or CFC products were causing these losses. Racking her brain for something that might be missing from the models, Solomon recalled an unusual phenomenon that occurs in Antarctica: high-altitude clouds of ice particles, called polar stratospheric clouds, that form in the ozone layer. Curious, she and colleague Rolando Garcia, a fellow atmospheric scientist, built an atmospheric model that included these polar clouds, with the ice particles providing a solid surface on which reactions could occur.


This seemingly small change in the hypothesis led to big changes in the results — the model was now predicting a large ozone loss. It looked like the presence of these tiny ice crystals made the destruction of ozone from CFCs much more efficient. With some preliminary results in hand, Solomon contacted Rowland. As it happened, Rowland was also wondering what would happen if solid surfaces were added into atmospheric models. Through laboratory experiments, he had already found that some key reactions (e.g., the release of destructive chlorine from ozone-friendly chlorine nitrate) occurred more readily on the surface of solids like glass and Teflon — and by extension, perhaps also ice from polar clouds.
Since Rowland was on a similar track to Solomon and Garcia, they decided to collaborate. With their proposed reactions, they explained how ice particles from the seemingly harmless clouds could not only free ozone-destroying chlorine, but also tie up the chemicals that could take chlorine out of commission, like nitrogen dioxide. The model they created with these reactions was able to match the Antarctic ozone observations, but more evidence was needed to determine if the ice clouds were really to blame for the extent of ozone destruction in Antarctica.