Starting in 1974, Mario Molina went on a campaign to end the use of chlorofluorocarbons (CFCs), a chemical commonly found in aerosol propellants and refrigerants. Molina had found evidence that they were contributing to ozone layer depletion. Use our science checklist to evaluate Molina’s work, and see how you think it measures up:

![]() Focuses on the natural world?
Focuses on the natural world?
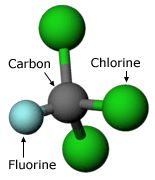
In 1973, Mario Molina began work with F. Sherwood Rowland at the University of California at Irvine. Rowland suggested that Molina determine the effects on the atmosphere of CFCs, human-made chemicals developed in the 1930s.
![]() Aims to explain the natural world?
Aims to explain the natural world?
Because there was no evidence that CFCs reacted with any common chemicals, it was assumed that they were safe for the environment. Since World War II, large amounts of CFCs had been released into the atmosphere, but no one actually knew what became of them. Molina’s work centered on finding out what happened to these CFCs and what their environmental consequences were.
![]() Uses testable ideas?
Uses testable ideas?
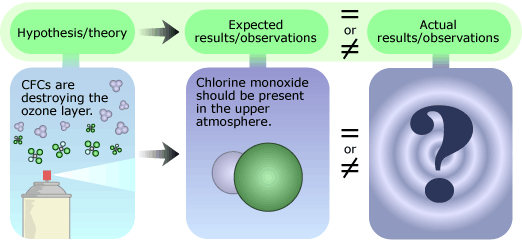
Molina’s research pointed to just one possible fate for the CFCs: they would remain in the atmosphere for a long time — somewhere between 40 and 150 years — before they drifted high enough for solar radiation to split off a highly reactive chlorine atom from the CFC molecule. This chlorine atom could then react rapidly with ozone, destroying the ozone molecule and depleting the ozone layer. Molina’s hypothesis consisted of three main points, each requiring it’s own tests:
- CFCs break down to produce chlorine. Scientists can recreate the intense solar radiation found in the upper atmosphere in their labs. Shining this lab-made solar radiation on CFCs and then looking for chlorine would provide evidence either for or against this idea.
- Chlorine atoms destroy ozone. Finding chlorine atoms and the products of the chlorine-ozone reaction in the ozone layer would support this idea.
- This is causing significant ozone depletion. The validity of this point could be examined by analyzing worldwide atmospheric ozone levels over time. Since CFCs remain in the atmosphere for so long before they break down, the effects might not be immediately measurable, and many years’ worth of monitoring might be needed to see how ozone levels are changing.

![]() Relies on evidence?
Relies on evidence?
Molina based his initial hypothesis on well-supported chemical theories and on previously published results, which showed that CFCs were accumulating in the atmosphere. He also used published results on the naturally occurring mechanisms that break down ozone to show that CFCs lead to an increased rate of ozone depletion. Molina left it up to other scientists to find additional atmospheric data. In 1985, atmospheric measurements showed that the ozone layer over Antarctica had been significantly depleted and indicated a continuing downward trend in ozone levels. By 1987, measurements had confirmed that the ozone loss was caused, at least in part, by chlorine, and in 1996, confirmed that CFCs were the key source of ozone-depleting chlorine.
![]() Involves the scientific community?
Involves the scientific community?
Collaboration with fellow scientist Rowland got Molina started on this project, and his initial hypothesis was based on the work of other scientists. Molina also shared his findings by publishing in the scientific journal Nature.1
![]() Leads to ongoing research?
Leads to ongoing research?
Because of its implications for the environment, Molina’s work inspired many other scientists to check and extend his results. Rowland started collecting air samples from all over the world to determine how fast CFCs accumulate in the atmosphere. James Lovelock considered other sources of chlorine and their effects on ozone depletion. S.C. Wofsy and others examined whether bromine, which is chemically similar to chlorine, could pose a similar threat. Molina’s work also encouraged an expansion of ozone measurements worldwide and the development of more sophisticated instruments to measure ozone and other chemicals.

![]() Researchers behave scientifically?
Researchers behave scientifically?
Molina reported his results accurately so that others could evaluate them. Furthermore, he acted with integrity by bringing his findings to the attention of the public and policy makers, so that something could be done to prevent the ongoing depletion of the ozone layer. Molina’s efforts, combined with evidence from ongoing research, convinced policymakers in 24 countries to phase out the use of CFCs, culminating in their almost complete elimination in 1996.
Now it’s up to you. How does Mario Molina’s investigation of the fate of CFCs in the atmosphere measure up to the science checklist?

Learn more about Mario Molina and Sherwood Rowland’s story in Ozone depletion: Uncovering the hidden hazard of hairspray.
1Molina, M.J., and F.S. Rowland. 1974. Stratospheric sink for chlorofluoromethane: Chlorine atom-catalyzed destruction of ozone. Nature 249:810-812.