During their investigation of cold fusion, Pons and Fleischmann tried to pull together scientific knowledge from chemistry and physics to produce something amazing — fusion at room temperature. To learn more about the science that factored into their reasoning, read on …
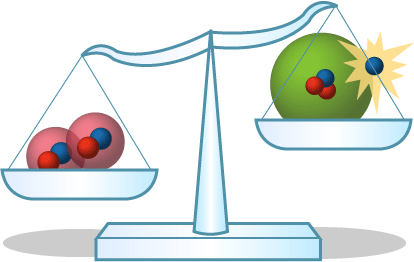
- Generating energy through fusion: Where does all the energy released during fusion come from? The answer lies in the mass. If you look at the mass of the reactants in the fusion reaction and compare it to the mass of the products, you’ll find that the two numbers aren’t equal — some mass has been transformed into energy, according to Einstein’s famous equation E = mc2.
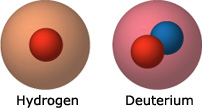
- Cold fusion “fuel”: Deuterium was the material Pons and Fleischmann chose to fuel their attempted fusion reaction. Deuterium is an isotope of hydrogen — meaning the two types of atoms have the same number of protons (one) but different numbers of neutrons: hydrogen has none, while deuterium has one. Since isotopes are so similar, they have nearly identical chemical properties — that’s why deuterium, like hydrogen, can be absorbed into palladium. Pons and Fleischmann used deuterium in their fusion experiments because deuterium fusion generates about 30 times more energy than hydrogen fusion does.
- Splitting water: Pons and Fleischmann used electrolysis to produce deuterium gas by splitting heavy water molecules — H2O in which the hydrogen has been replaced by deuterium. When an electric current is run through the heavy water in a fusion cell, it triggers chemical reactions at the metal rods. Lithium deuteroxide (LiOD) was added to the water to increase its ability to conduct electricity and speed up the process. At the palladium rod, the heavy water (D2O) reacts with the electrons that make up the current to produce deuterium gas and deuterium oxide ions:
2D2O (l) + 2e– › D2 (g) + 2OD– (aq)
At the platinum rod, the deuterium oxide ions react with each other to produce oxygen gas, water, and electrons:
4OD– (aq) › O2 (g) + 2D2O (l) + 4e–
So in the net reaction, water decomposes into oxygen and deuterium gasses:
2D2O (l) › O2 (g) + 2D2 (g)
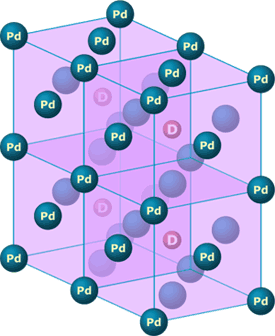
- Palladium-deuterium interaction: Palladium is actually a crystal — meaning that its atoms are organized in a pattern that repeats itself over and over again to make up the solid. The basic pattern underlying palladium is a cube with an atom at each corner and at the center of each face of the cube. When deuterium is absorbed into palladium, it is drawn into the center of these cells and can then diffuse through the crystal, hopping from cell center to cell center. At such a small scale — a length of about 1/100,000th of the width of a strand of hair — quantum mechanical effects are important. Being chemists who didn’t specialize in quantum mechanical phenomena, Pons and Fleischmann thought that perhaps the quantum mechanical interactions between the palladium atoms and the deuterium atoms could cause the deuterium atoms to get close enough to fuse. Physicists later showed that this was not the case.
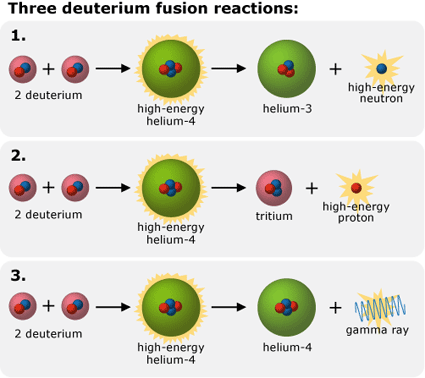
- Fusion products: According to nuclear theory, when two deuterium atoms fuse, they form highly energetic helium-4 (a two-proton, two-neutron atom). The helium-4 then releases some of its energy in one of three ways:
-
- ejecting a proton and transforming itself into tritium (an isotope of hydrogen with two neutrons and one proton)
- ejecting a neutron and transforming itself into helium-3 (an isotope of helium with one neutron and two protons)
- releasing a gamma ray and remaining helium-4 (but with much less energy)
These particles will then collide with molecules in their surroundings and thus transfer some of their energy to the palladium and water as heat.
- Detecting fusion products: Techniques had already been developed to detect each of the six fusion products (protons, neutrons, gamma rays, tritium, helium-3, and helium-4) — but Pons decided to concentrate on searching for neutrons since they were expected to be relatively abundant among the products and were the easiest to detect. When Pons’ initial, direct search for neutrons came up empty, he began to wonder if something was happening to them before they could reach the detector. If neutrons were being created in the fusion cell, then they would end up traveling through the water surrounding the cell. Perhaps the neutrons were reacting with the water before they could be detected. So in his second attempt to find evidence of neutrons, Pons looked for the product of the neutron-water interaction — gamma rays. These gamma rays would be different from the gamma rays produced by the fusion event itself because these secondary gamma rays would have much less energy. Some of the secondary gamma rays would then interact with the water in a way that would have been detectable. Pons’ neutron evidence was ultimately discredited because there was no sign of this interaction between the secondary gamma rays and water.