Fleischmann filed away his ideas about fusion until the fall of 1983, when he and Pons started talking about the possibility of using chemical processes (reactions among atoms and molecules) to trigger a nuclear process (changes within the nuclei of atoms). They decided to set up a full-blown experiment to test Fleischmann’s idea. Working in Pons’ laboratory, the two put together what they called a “fusion cell.” This cell consisted of two pieces of metal, one palladium and the other platinum, submerged in a container of heavy water (water in which the hydrogen of each H2O molecule is replaced by deuterium). They knew that if they zapped the cell with electricity it would trigger a chemical process called electrolysis, in which the heavy water molecules would split, producing deuterium gas and oxygen. The deuterium could then be absorbed into the palladium via a chemical reaction. Pons and Fleischmann hypothesized that, once inside the palladium, the deuterium atoms would be forced so close together that they would fuse and release large amounts of energy as heat.
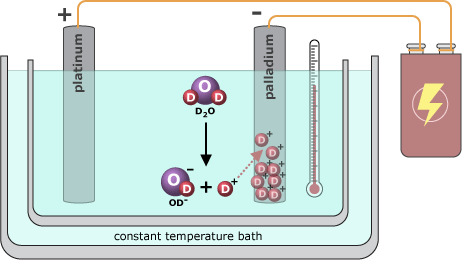
Pons and Fleischmann measured the temperature of the cell continuously throughout its operation. After some analysis of the data, they found that the cell was producing about 100 times more heat than could be accounted for by chemistry alone! They interpreted this excess heat as evidence for fusion. Excited by the possibility that they had found an inexpensive way to harness fusion for energy production, Pons and Fleischmann were eager to test their idea further. However, more experiments required more money …
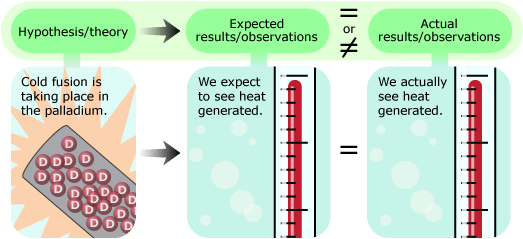
Pons and Fleischmann’s fusion cell was simply a standard chemistry lab device known as an electrolytic cell. Since this topic is commonly addressed in high school or middle school chemistry classes, it offers an opportunity to connect this story with the science your students are experiencing in class. To get directions for a simple demo, visit ScienceFix.